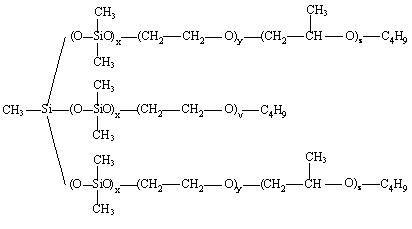
Surface-active materials are essential in the manufacture of most PU. They are particularly useful in foam making where they: lower surface tension; emulsify incompatible formulation ingredients; promote nucleation of bubbles during mixing; stabilize the rising foam by reducing stress concentrations in thinning cell-walls; and counteract the defoaming effect of any solids added to or formed (e.g., precipitated polyurea structures, during conventional flexible foam reaction). Early polyester polyurethane foam systems used one or more organic, usually non-ionic surfactants. Some polyester based low-density flexible foams and some semi-rigid foams can be produced using these organic surfactants such as substituted nonyl phenols, fatty acid ethylene oxide condensates and alkylene oxide block copolymers. For the manufacture of polyester foams surface-active agents containing sulfonic acid groups (as sodium salt of sulfonated ricinoleic acid or Turkish red oil) have proved to be good foam stabilizers. However, nowadays most flexible and rigid foams are made using organosiloxanes or silicone based surfactants. The first silicone polymers to be used in the production of PU foams were poly(dimethylsiloxanes) (PDMS) and poly(phenylmethylsiloxanes). These materials remain of value in some flexible and semi flexible foam systems, but the majority of low-density foams are now made using PMDS-polyether graft copolymer surfactants (Figure 2.10) that have been developed specially for the purpose.

Figure 2.10 - Structures of PMDS-polyether graft copolymer surfactants
2.4.1 Selection of the silicone surfactant
To meet the surfactant needs for the PU formulation and of the application technique the surfactant structure may be modified by changing the length of the PDMS hydrophobic backbone, the number, length and the composition (the oxyethylene (EO) / oxypropylene (PO) ratio) of the pendent hydrophilic polyether chains. The polyether is capped to ensure a good mobility of the surfactant.
|
Flexible PU foam |
Conventional |
HR |
Ester |
|
% de siloxane |
15 – 35 |
50 - 100 |
3 – 30 |
|
% EO in polyether |
35 – 55 |
0 - 100 |
75 – 100 |
|
Molecular Weight |
5.000 - 35.000 |
500 - 1500 |
500 – 1500 |
|
End group on the polyether chain |
OR* |
Cl, CN, OR**, OH |
OR, OCOOH |
A number of different variables can be used in the silicone surfactant design to cover any application demand. A higher EO content in the polyether chains and more polyether increases the water solubility. A longer polyether chain increases the foam stability. A long siloxane backbone increases the stability as well. Conventional polyether slabstock foams are difficult to stabilize due to the low reactivity (even with tin gelling catalysts) of polyol with 80-100% of secondary hydroxyl groups. This means that significant amounts of blowing agent evaporation take place before the PU network formation is complete. The foam expands rapidly and the rising froth must be able to support its own weight, yet the strength of the polymer is limited until late in the foam process, and the surfactant must stabilize the foam over relative long time periods while the cure is proceeding. Consequently, conventional polyethers systems require good stability and a good processing latitude and this is achieved with high MW siloxane / high MW polyether, and high EO respectively. The surfactants employed for making conventional flexible polyether foams have high MW (20,000 to 80,000) with longer grafted polyether chains with a higher polyoxypropylene content.
|
CONVENTIONAL ESTER HR |
|
Polyester systems are more reactive and stable than the conventional ether systems, and will require lower MW silicone surfactants. They should provide a very regular cell structure through a good emulsification of air and components. Polyester based flexible foams require surfactants of lower activity with MW ranging from 500 to 1,500 and much shorter polyether chains.
The HR systems are stable due to the high reactivity of the polyols and the presence of crosslinkers. The critical issue here is cell openness and structure more than stability. Therefore, silicone surfactants with low MW siloxane and low MW polyethers are most commonly used. Typically flexible molded surfactants are the smallest with molecular weight varying from 300 to 1,500. Table 2.8 shows the composition requirements specified for flexible PU foams, and relative non-hydrolysable structures for flexible foam are represented in Figure 2.11.
Finally, silicone surfactants for rigid foams have a greater surface activity than those for flexible foam. They have MW raging from 1,500 to 15,000 with predominantly hydrophilic polyoxyethylene polyether chains pendent from the hydrophobic PDMS.
Depending on the structure, the polyethersiloxanes are hydrolysable (Figure 2.12) or non-hydrolysable (Figure 2.13).
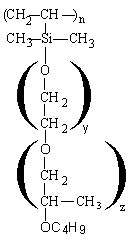
Figure 2.12 - Hydrolysable silicon surfactant (Si-O-C bonding)
The Si-C bonding is non hydrolysable while Si-O-C bonding is hydrolysable. For the foam, the stabilizing influence of the Si-O-C hydrolysable bonding is unimportant. However, mixtures of silicone surfactant, tertiary amines and water are frequently stored for a long period of time. Under this conditions the Si-O-C bonding can be hydrolyzed and the products loses its foam stabilizing properties.
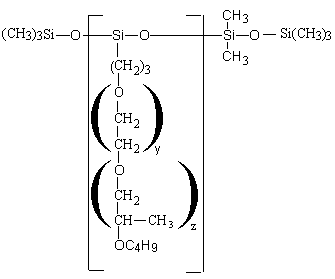
Figure 2.13 - Non-hydrolysable silicon surfactant (Si-C bonding)
2.4.1 Low density foams stabilization mechanisms
Surfactants are molecules that have built-in one-structure characteristics that allow them to bridge the property differences between two materials or different properties. Typically, one component in the surfactant has an affinity for no polar phase (hydrophobic) and the other an affinity for polar phase (hydrophilic). Surfactants orient themselves in the interfacial region between the two phases. During the formation of PU foam it is believed that the silicone surfactant functions at four discrete phases: dispersion of reactants, nucleation, stabilization, and cell opening in flexible PU foams (Figure 2.14). All of these activities have a major impact and affect the ultimate physical properties of the final foam. Silicone surfactants play a role in the mixing, during the laydown process and during the rise: 1) Emulsification of chemicals with air, by decreasing the surface tension of the system, which facilitates the air mixing and distribution; 2) Emulsification / compatibilisation of the reaction mixture; 3) Control of the drainage of the material from the bubble walls, prevention of cell coalescence during foam rise.
Ø Dispersions of reactants - The first step in the foam making process is to combine several different ingredients, some of which are incompatible with one another. The silicone-polyether surfactant is soluble in the PU intermediates so that it functions as an emulsifier promoting efficient mixing. The degree to which the surfactant compatibilises these reactants depends upon the reactive amount of silicone and polyether it contains, and in the relative number of ethylene and propylene oxide units in the polyether. More polyether in the surfactant and more ethylene oxide in the polyether give copolymers with increased water solubility. Good emulsification of the reacting mixture contributes to superior flowability, and also improves blowing agent utilization, resulting in lower foam densities. Commercial box foaming is particularly challenging due to the high levels of both water and methylene chloride. Liquid carbon dioxide blown foams are more challenging still since vaporization is extremely rapid once the reactants are poured at atmospheric pressure. Both technologies requires superior emulsification due to high use levels of poorly compatible blowing agents, while considerable stabilization for rapidly expanding froth is required in the foaming process.
Ø Nucleation - Bubbles of air are introduced during the mixing process of the foam ingredients. In the absence of any surfactant the volume of air introduced is very small. The bubbles are fewer and larger as coalescence takes place rapidly without the stabilizing influence of the surfactant. It has been demonstrated that all the cells of the final foam are present as small air nuclei before the foam begins to rise. The number and size of air nuclei entrained in the reactants mixture is determined by the mechanical energy of the mixing procedure and the surfactant. The surface activity of the surfactant increases the volume of air nuclei mixed into reactants and decreases the tendency of gas to diffuse from smaller bubbles to larger ones. This results in foam with finer, more uniform cells.
Ø Bubble growth - Several mechanisms lead to the cell growth: 1) Diffusion of newly formed gas into existing bubbles. The reaction between water and isocyanate produces carbon dioxide. Simultaneously, the heat of reaction vaporizes the liquid blowing agent. Very rapidly the solution becomes supersaturated with gas. At this point, the dissolved gas begins to come out of the solution. However, a limited number of air bubbles (air-liquid interface) limits the rate at which gas may diffuse from the liquid to the air bubble; 2) expansion of gas in the bubbles due to heat of reaction; 3) diffusion of gas from smaller bubbles into larger bubbles. A low surface tension favors the reduction of the pressure difference between bubbles of different size. This decreases diffusion thus promoting smaller average cell size.
Ø Bubble coalescence - Bubble coalescence occurs when the liquid separating two bubbles beaks. This phenomenon is related to bubble stability.
Ø Bubble stabilization - After bubbles are formed in the developing foam system, these bubbles must be stabilized until the cell structures attain structural integrity through polymerization. A thinning area on the common wall of these bubbles due to temperature, drainage, and capillary action can lead to a rupturing of the cell wall. This effect can propagate to other cells thereby producing a split in the foam or total collapse. The surfactant must operate to make the liquid draw toward the thinned area, and restore the original thickness of the cell wall (Figure 2.15). The surfactant functions to stabilize or reduce surface tension gradients (thinning in the cell walls) by allowing the surface layer to diffuse from areas of lower to those of higher surface tension, thus restoring the film to its original thickness.
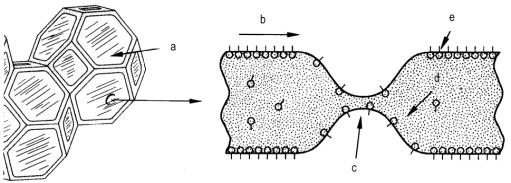
a) intact cell window; b) surface transport; c) thin area with rupture danger;
d) bulk transport; e) surfactant molecules
Figure 2.15 - Thinning in a cell window
Ø Cell opening - Near the completion of foam rise, the spherical bubbles of the foam matrix are closely packed polyhedrals. The flat faces of these polyhedrals are thin membranes, which interest at struts. Capillary drainage and drainage due to gravity results in a flowing back of the membrane into the struts. In order to avoid shrinkage in a cured flexible moulded or slab foam, drainage of the membranes must coincide with complete foam expansion and some degree of polymerization to make the struts self supporting as the expanding gas escapes. On the other hand, in rigid PU foams, when the polymerization is complete, the cells must be closed. At this stage, the cell walls must resist the gas internal pressure.
Microcellular systems and RIM formulations consist of basic polyol, which are a difunctional and/or trifunctional polyether or a linear polyester, a short-chained crooslinker, a catalyst and a blowing agent. The isocyanates are based on MDI, and they are difunctional or carry urethoneimide and/or carbodiimide modifications. Normally these materials react in two steps: first, the short-chained crosslinker reacts with the isocyanate forming hard segments; then, the reaction between the polyol and the isocyanates starts, resulting in the formation of soft segments which link up with the hard segments, forming a domain structure. If rather compact foam parts have to be produced, this kind of system can be processed without adding any blowing agent intentionally. Processing these systems results in the formation of microcellular structures. The shape of the cells in these structures is mainly spherical when the foam density is above 250 kg/m3. Bellow this, cell structure becomes polyhedric and therefore thermodynamically more unstable.
Ø Nucleation step - To obtain a regular finely celled foam one needs many small gas bubbles or nucleation centers in the reaction mixture; i.e. the air mixed into the systems has to be dispersed very finely, and homogeneously. As it happens with the low-density foam systems, even at high gas super saturation conditions additional self-nucleation does not occur. In other words, all the cells of the final foam must already exist as gas bubbles at the beginning of the foaming processes.
Ø Expansion stage - The blowing gas generated by simple evaporation or chemical reaction between the isocyanate and water forming carbon dioxide, migrates into the nuclei, which have been formed by the mixing process. This is the stage of expansion where the polymerization starts simultaneously. During this stage, the foam is stable, and in most cases, an additional physical stabilization provided by a surfactant is not required. The shape of the bubbles remains spherical until the gas volume of the system reaches about 75% of the total volume. At this point, which correspond to the density of 250 kg/m3 the geometrical arrangement of the bubbles represents approximately hexagonal dense packing in the liquid matrix. When the relative gas volume exceeds this critical value, the spherical foam is transformed into a polyhedral system (Figure 3.5 - Chapter 3), where the cells are built up of the struts and thin membranes.
Ø Cell regulating - Surface-active materials, useful for this application, are short-chained dimethylsiloxanes. Those siloxanes are very mobile and, due to their low surface tension, they quickly cover all surfaces of the incompatible liquids and of the tiny air bubbles, and spread on the internal surfaces. This is normally just an intermediate effect. It has to last until the reaction has formed its own emulsifying species and crosslinking takes over the role to stabilize the bubbles additionally. The effect of a cell regulating surfactant is recognized in the final foam in a finer and more regular cell structure. This also improves properties like firmness and fatigue and, in shoe soling, the Ross-flex-results. Cell regulators are applied in systems for integral skin foam and for ether and ester based shoe soles. However, in the production of polyester based midsoles in densities of about 250-350 kg/m3, the use of the cell regulating additives can form a more closed cell structure and shrinkage.
Ø Air dispersing - In some RIM systems with high reactivity and no blowing agent, there is a need of an air load of 40-50% in the recirculation stream of the machines. This generates an internal pressure in the mould after the injection of the liquid mixture, necessary to avoid sink marks and pinholes. These defects will appear whenever the internal pressure diminishes too quickly. Chemically, nucleating additives are polyether-modified dimethylpolysiloxanes, which act like cell regulating additives, and disperse the air mixed in the system. This fine dispersion is necessary for dissolving the gas in case of increased pressure. So, by means of the silicone surfactant the amount of gas entrapped in the system is controlled and stays high due to the high pressure at the outlet. The dissolved / entrapped air in the system causes a pressure build up in the molded part, during the time in the mold and after demolding. So sink marks are avoided and improved dimension stability is achieved.
Ø Flow promoters - Improved flowability in expanding, still liquid PU mixture is required for moulds of geometrically complicated shapes with long flow-paths and narrow notches as in the case of glycol based RIM systems or integral skin foams of high density. The addition of appropriate organo-modified siloxanes to the resin component makes it possible to achieve an increased number of air-nuclei, and to stabilize the air in the recirculation stream. It is very important that the dispersion of the air in the polyol is stable to avoid coalescence too early when the mixture has to pass baffles or narrow notches. By using flow promoters, it might be possible to save some raw materials, and to reduce the weight of the parts.
Ø Improve mixing - Surfactants make compatible the no miscible ingredients of the resin side of a formulation when it is being blended, and when the polyol and the isocyanate components meet in the mixing head, and have time to separate in the mold during long pot life (e.g. cast elastomers). Casting PU may show inhomogeneities, which will disappear if dimetylpolysiloxane or polyetherpolysiloxane copolymer products are added.
2.5 - Fillers