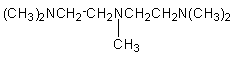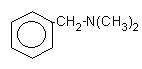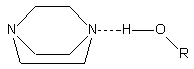Tertiary amines are
the most widely used catalysts in the manufacture of cellular and solid PU's.
Some of the more commonly used tertiary amines are shown in Table 2.3. In PU
foams, tertiary amines control the blowing and polymerization reactions, and
play a relevant role in PU end properties.
Table 2.3 - Tertiary amine catalysts
|
Catalyst
|
Application
|
|
1.
N,N-dimethylethanolamine (DMEA)
(CH3)2NCH2CH2OH
|
Inexpensive, low-odor, isocyanate
reactive and blowing catalyst used in polyether flexible foams.
|
|
2.Diaminobicyclooctane
(DABCO) or triethylene diamine (TEDA)
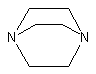
|
General-purpose gelling catalyst,
solid crystal soluble in water, glycols and polyethers polyols.
|
|
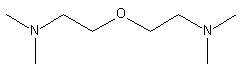
3.
bis(2-dimethylaminoethyl)ether (BDMAEE)
(CH3)2NCH2CH2OCH2CH2N(CH3)2
|
Low odor, strong blowing catalyst
used in flexible foams.
|
|
4.
N-ethylmorpholine
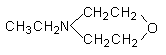
|
Low odor, used in polyester foams,
and additionally improve the skin formation.
|
|
5.
N'N'-dimethylpiperazine

|
Based on the high vapor pressure is
used to improve the skin formation in molded foam.
|
|
6.
N,N,N’,N’,N’’-pentamethyl-diethylene-triamine (PMDETA)
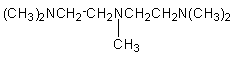
|
Highly active blowing catalyst for
flexible, semi-rigid and rigid foams.
|
|
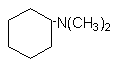
7.
N,N-dimethylcyclohexylamine (DMCHA)
|
Liquid with intense odor used in rigid
foams, leads to a well-balanced proportion of gelling and blowing reactions.
|
|
8.
N,N-dimethylbenzylamine
(DMBA)
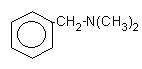
|
Liquid
with smell characteristic employed in polyester flexible foams for reduced
scorching, semi-rigid and in rigid foams for small cell size and good
adhesion.
|
|
9. N,N-dimethylcethylamine
CH3(CH2)14CH2N(CH3)2
|
Viscous liquid with a low odor used
in polyester based flexible foams and some potting compounds.
|
|
11.
N,N,N’,N”,N”-pentamethyl-diproylene-triamine (PMDPTA)
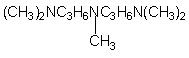
|
Strong gelling catalyst with good
flowability, strong ammoniacal odor used in polyether based slabstock
foams, semi-rigid, and rigid foams.
|
|
12.Tritehylamine
N-(CH2CH3)3
|
Highly volatile amine catalyst. Acts
as a surface cure catalyst and reduce defects associated with lower temperature
molds. Balanced blow and gel for molded and slabstock foams.
|
|
13.1-(2-hydroxypropyl)
imidazole
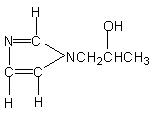
|
Isocyanate reactive and gelling catalyst
for polyether based foams and low-density rigid foams.
|
In agreement with its chemical behavior, flowability,
and PU end properties, tertiary amines may be classified as: Gel catalysts,
Blow catalysts, Delayed action catalysts, Skin cure catalysts, and Reactive
catalysts.
Gel catalysts
- Gellation catalysts promote the
polyaddition reaction of isocyanate with polyol. These catalysts are non-sterically
hindered tertiary amines having the free electronic pair available on the nitrogen
atom; they activate the C=N moiety of the isocyanate group. This activated complex
quickly reacts with the active hydrogen atom of the polyol, forming the PU.
DABCO or TEDA is a strong general-purpose gel catalyst, due to the absence of
steric hindrance, otherwise, dimethylcyclohexylamine (DMCHA) is sterically hindered
but it is a strong base, widely used in PU rigid foams.
Blow
catalysts - These
catalysts promote the blowing reaction between the isocyanate and water, forming
polyurea and carbonic gas, which acts as a blowing agent. They are non-sterically
hindered tertiary amines with ethylene groups between the two (nitrogen or oxygen)
active centers. These two active centers are able to chelate water (Figure 2.6a),
thus increasing its reactivity.
Delayed
action catalysts - Foam
flowability is very important to properly fill the mold, especially with large
and complicated profiles. On the other hand, good curing is essential to perform
short demolding times and to avoid fingerprinting when the pad is taken out
of the mold. To combine excellent in-mold flowability and fast curing, delayed-action
catalysts were developed. When starting reactivity is too high, delayed-action
blow catalysts should be employed; on the other hand, delayed-action gellation
catalysts are used to delay the viscosity build-up of the reacting mixture allowing
to flow easily in the mould cavity. The process to manufacture a delayed-action
catalyst involves reacting a specified tertiary amine with a carboxylic acid.
The resulting compound is composed of a salt and an excess of the starting amine.
The salt has limited or no catalytic activity and when this blend is used in
a foam formulation, the free amine launches the reaction, but when the reactions
have progressed, with heat generation, the salt dissociates to yield back the
amine and the acid. At this time the catalyst is de-blocked and has recovered
the original activity. The released organic acid reacts with isocyanate-forming
carbon dioxide and carbon monoxide, supplying an auxiliary source of blowing
agent, resulting in less dense with more open cell foams. Normally, carboxylic
acids such as formic and 2-ethylhexanoic acids are used as blocking agents.
The unblocking temperature depends on the acid used. Strong acids require higher
temperatures than the weak ones. In commercial applications the unblock temperature
is located between 30oC and 60oC. There is some corrosion linked to the use
of formic acid and the foams produced tend to be very closed and are therefore
difficult to open.
Skin cure catalysts - The
skin cure catalysts provide additional isocyanate reaction at the surface, improving
the overall appearance of the part and eliminating defects such as fingerprint.
There are two categories of surface cure catalysts. The first includes tertiary
amines that have high vapor pressure and volatilize from the developing foam
to the foam mold surface where they provide additional reactivity. Typical examples
of these catalysts include triethylamine (TEA), N-methylmorpholine (NMM), and
N-ethylmorpholine (NOR). The second group includes tertiary amines that are
incompatible with the developing foam and migrate to the mold surface and provide
the same effect as above. Typical amines of this category are modified morpholines.
Reactive
catalysts - Reactive
amine catalysts, as DMEA, DMAEE and TMAEE that contain a hydroxyl group, are
able to react with isocyanates becoming chemically a portion of the polymer
matrix. The disadvantage of polymer-bonded catalysts may be the deterioration
of foam physical properties, especially when the foam is exposed to hot and
humid climates. They are used in special applications, such as dashboard production,
in order not to migrate into the PVC skin, this causing its discoloration.
2.1.2.1
- Catalysis mechanism
Since Otto
Bayer's pioneering work, the catalysis mechanism of isocyanates reactions with
alcohols (gel reaction) or with water (blow reaction) was studied by several
authors and especially by Baker and Farkas. Baker postulated the formation of
a complex consisting of an isocyanate and a tertiary amine catalyst, followed
by the attack by nucleophilic reagents (water or alcohols) (Figure 2.2).
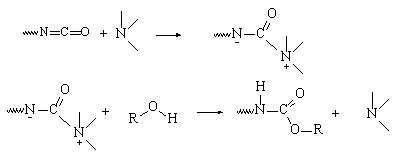
Figure
2.2 - Tertiary amines catalysis Baker's mechanism
Farkas based his theory
on the initial formation of a complex between the nucleophilic reagent (alcohol
or water) and the tertiary amine (Figure 2.3). This complex then reacts with
the isocyanate, and the gel reaction (with polyol), or blow reaction (with water)
occurs, with regeneration of the tertiary amine. In this mechanism the amine
basicity is the predominant factor.
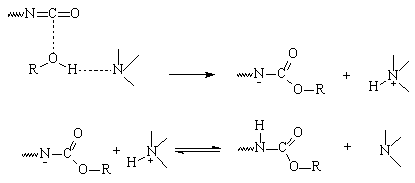
Figure
2.3 - Tertiary amine catalysis Farkas' mechanism
2.1.2.2
- Gel and blow catalysis
Alcohol
interacts with tertiary amines like TEDA (triethylenediamine) through a single
hydrogen bond (Figure 2.4).
Figure
2.4 - Interaction of water (R=H) and alcohol with TEDA
Water is able to interact
with tertiary amines through different modes. Water interacts with TEDA (a strong
gelling catalyst) through a single hydrogen bond like an alcohol. On the other
hand, tertiary amines of higher blowing activity such as BDMAEE [bis(2-dimethylaminoethyl)ether]
or PMDETA (N,N,N',N',N''-pentamethyl-diethylenetriamine) (Figure 2.5) are able
to chelate water (Figure 2.6). This indicates that a single nitrogen-water hydrogen
bond does not result in effective catalysis of the blowing reaction. The water-chelated
complex, which is not feasible with TEDA, may then be the selective high-activity
catalyst precursor.
Figure 2.5 - BDMAEE and PMDETA structures
PMDPTA (N,N,N',N',N"-pentamethyldipropylenetriamine)
is also unable to chelate water (Figure 2.6). Water chelation by the central
nitrogen and one of the terminal nitrogens is disfavored by close contact between
water hydrogens and hydrogens form the three-carbon bridge. These different
modes of water binding provide an explanation for the experimental observation
that acyclic tertiary amines with hetero atoms separated by a two-carbon bridge
favor the blowing reaction more than tertiary amines with hetero atoms separated
by a three-carbon bridge.
BDMAEE
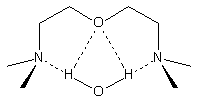 |
|
PMDPTA

|
Figure 2.6 - BDMAEE and PMDPTA water binding
Table 2.4 includes the catalytic
activity of many tertiary amines. The gelling and blowing catalytic activities
were measured by a titration method in a standardized reaction system containing
TDI, diethylene glycol and water. TEDA is a strong gelling catalyst. BDMAEE
and PMDETA are strong blowing catalysts. TMHMDA, TMEDA and DMAEMP exhibit moderate
activity between the gelling and blowing reactions. ROCONHC6H6 and R'NHCONHC6H6
are reactive tertiary amines.
Table 2.4 - Reaction rate constant and activating
energies of amine catalysts
|
Name
|
Abreviation
|
Gelling activity (k1)
|
Blowing activity (k2)
|
Ratio blowing/gelling
|
| |
|
(x 10)
|
(x 10)
|
(x 10-1)
|
|
Triethylenediamine
|
TEDA
|
10,90
|
1,45
|
1,34
|
|
TEDA 33%
in DPG
|
|
3,63
|
0,48
|
1,34
|
|
|
TMHMDA
|
2,95
|
0,84
|
2,85
|
|
N,N-dimethyl
cyclohexylamine
|
DMCHA
|
2,22
|
0,83
|
3,76
|
|
N-(2-dimethylaminoethyl)-N’-methylpiperanize
|
DMAEMP
|
1,71
|
0,78
|
2,72
|
|
N,N,N’,N’-tetramethylethylene
diamine
|
TMEDA
|
4,19
|
1,14
|
2,72
|
|
N,N,N’,N’,N”-pentamethyldiethylene
triamine
|
PMDETA
|
4,26
|
15,90
|
37,30
|
|
Bis(2-dimethylaminoethyl)
ether
|
BDMAEE
|
2,99
|
11,70
|
39,00
|
|
BDMAEE 70%
in DPG
|
|
2,09
|
8,19
|
39,00
|
|
N,N-dimethyllaminoethanol
|
DMEA
|
2,91
|
0,36
|
1,23
|
|
N,N-dimethylaminoethoxyethanol
|
DMAEE
|
1,84
|
2,55
|
13,90
|
|
N,N,N’-trimethylaminoethyl
ethanolamine
|
TMAEEA
|
2,89
|
4,33
|
15,00
|
|
Imidazol
based catalyst
|
|
3,54
|
0,39
|
1,10
|
|
Imidazol
based catalyst
|
|
2,69
|
0,20
|
0,74
|
|
ROCONHC6H6
|
|
2,36
|
0,27
|
1,14
|
|
R’NHCONHC6H6
|
|
2,46
|
1,00
|
4,01
|
2.1.3
- Organometallic catalysts

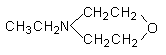
![]()