
a - urethane linkage
b - polyol chain
PUs molecular structure can vary from rigid polymeric (crosslinked), to linear, elastomeric flexible chains (Figure 1.35). Flexible foams and TPU elastomers have segmented structures, made up of long flexible chains (polyols) linked by polyurethane and polyurea aromatic hard segments. Their character depend largely on hydrogen bonds between polar groups of the polymeric chain, mainly among N-H groups (electron acceptors) and carbonyl groups (electron donors) of urea and urethane groups. Hydrogen bonds can also be formed among N-H groups and polyester carbonyl groups and, more difficultly, with polyether oxygen atoms (weak bonds). Hard segments of flexible PUs, especially of polyurea, form strong secondary chemical bonds (hydrogen bonds) with a tendency to form hard segment domains. On the other hand, as a result of the polyfunctional reagents used, rigid PU foams are highly crosslinked, and they don't show the segmented structures present in flexible PUs. Besides urethane bonds, the PUs macromolecular chain possesses a multiplicity of other groups that contribute to the cohesive macromolecular forces (Table 1.12).
Table 1.12 - Cohesive molar energy of organic groups|
Organic group |
Choesive molar enrgy (kcal/ml) |
|
-CH2- (hydrocarbon) |
0,68 |
|
-O- (ether) |
1,00 |
|
-COO- (ester) |
2,90 |
|
-C6H4- (aromatic ring) |
3,90 |
|
-CONH- (amide) |
8,50 |
|
-OCONH- (urethane) |
8,74 |
a) Soft, high elongation elastomers
 |
a - urethane linkage b - polyol chain |
b) Polymers with a segregated domain structure (high modulus elastomers and flexible foams)
 |
a - hard block domain b - soft block domain |
c) Rigid, highly cross-linked polymers
 |
-
= diurethane linkage T= triurethane linkage + = tetraurethane linkage * = polyurethane linkage |
Figure 1.35 - Polyurethane structures
1.6.1 - Segmented polyurethanes
Properties of PUs produced from 1,6-hexane diisocyanate and 1,4-butane diol are like those of polyamides of similar structures. Amorphous PUs (prepared with TDI and diethylene glycol) are rigid and transparent, but they show low dimensional stability at high temperatures. On the other hand, there are PUs formed exclusively by soft segments, obtained by the stoichiometric reaction of a difunctional polyol with diisocyanate, resulting in amorphous products with elastomeric properties. Here, intermolecular forces are essentially between the polyol soft segments so that properties such as hardness and mechanical resistance are poor. All these products have a single phase and they don't present segmented structures. The only non-segmented PUs of commercial importance are the highly cross-linked PUs such as rigid foams and non-textile coatings. Segmented PUs is formed by the reaction of a polyol, diisocyanate and a chain extender, which can be a glycol, diamine or water. These PUs represent a class of products, characterized by a segmented structure (polymeric blocks) made up of two or more different polymeric phases. These segmented structures are responsible for the excellent PUs properties.
Hard and soft segments
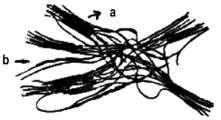
A PU prepared with one mole of long chain linear polyol [poly(1,4-butane diol adipate)], two moles diisocyanate (MDI) and one mole chain extender (1,4-butane diol) presents the structure shown in Figure 1.36. Soft segments, which are quite mobile and are normally present in a coiled shape, and hard segment units, alternate.
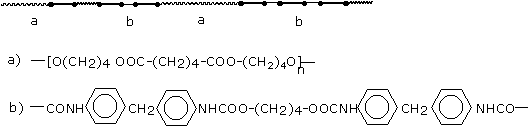
a) sof segments, b) hard segments
Figure 1.36 - Representation of a segmented PU chain
Morphology of the segregated domains
Usually, Pus' soft segments are incompatible with the hard and polar ones. As a consequence a phase separation (segregation) occurs and covalently linked microphases are formed. The coherent matrix, which consists of flexible soft segments, results in high deformability of the resulting material. In contrast, within the hard segment domains, molecules are fixed by physical interaction. Because of covalent coupling to the soft segments, they inhibit plastic flow of the chains, thus creating elastomeric resiliency. Hard segmented domains can be looked at as multifunctional spacious crosslinked areas. The larger the phase segregation, the lower the polarity of the flexible segments. Therefore, segregation is less pronounced in polyester urethanes compared to polyether urethanes and is most pronounced in polybutadiene urethanes (Figure 1.37).
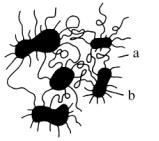 |
a - soft block domain b - hard block domain |
Figure 1.37 - TPU structures
Hard domain morphology
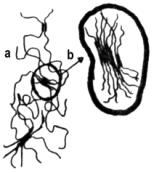
Depending on the nature and length of the hard segments and the degree of segregation, three-dimensional organized proximity areas are formed with structures of predominantly paracrystalline nature. In case of very low cooling and sufficient length of the hard segments, even microcrystallites can be formed (Figure 1.38). The secondary structure depends on the proximity zone interaction between hard segments. This structure is mainly characterized by hydrogen bonding between adjacent aromatic rings of symmetrical isocyanates. Another important interaction is that existing between p electrons of the isocyanate aromatic rings.
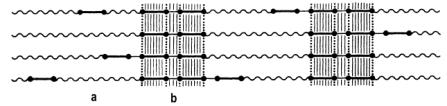
a) sof segments, b) hard segments
Figure 1.38 - Interchain interaction between hard segments
1.6.2 - Effect of hard segments
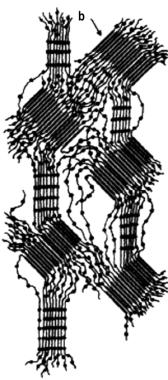
Interactions between chains, mainly hydrogen bonds between hard segments, contribute to the distinguishing properties of PUs. Thermo-mechanical properties of linear segmented PUs are substantially different from those of chemically crosslinked products. Whenever mechanical forces are applied, changes in the orientation and mobility of structures within hard segment domains, which depend on temperature, can occur. In this process initial hydrogen bridges are broken and other, energetically more favorable, are formed. A change in structure occurs, causing alignment in the direction of the applied tension (Figure 1.39). As a consequence, applied tension is better distributed and as a result, the resistance of the material against further stress is increased. This effect contributes to the high tensile strain, elongation, tear strength, and permanent set values.
The melting range of the hard segment domains determines the dimensional thermal stability of linear segmented PUs. Above the melting range the material is thermoplastic. With the increasing length of the hard segment the melting range also rises and with the use of different chain extenders and isocyanates the melting range can be intentionally modified (Chapter 6.3). Above the melting range of the hard segments, linear PU's form a viscous homogeneous liquid that can be processed as a thermoplastic material. Whenever the melting range is above 250oC (which means higher than the PU decomposition temperature), even linear PUs will not exhibit thermoplastic properties. Upon increasing the amount of hard segment, PU shows an increase in hardness and modulus, and above 60% by weight there is a change in behavior from elastomeric to a brittle, high-modulus plastic.
|
Schematic representations of PU linear segmented structures |
||
|
I -Relaxed structure
(without stress) |
II - Structure with 200% elongation |
III - Structure with 500% elongation |
 |
 |
 |
|
a) soft segment, b) hard segment, c) reorientation after stress |
||
Chain mobility largely depends on the chemical nature and size of soft segments. Soft segments control properties such as cold flexibility, as well as PU chemical behavior, such as resistance to solvents, water, acids, bases and weather. In order to obtain suitable elastomeric properties, especially impact resistance, the soft segment should be amorphous and possess a low enough glass transition temperature. To prepare PUs with typical rubber elasticity an average molecular weight of 1,000 to 4,000 is desirable, corresponding to a chain length of 120 to 300 A. Whereas the elastomers freezing temperature TE (lower end of the glass transition) is about 20 to 30oC above that of the polyol used, T* (the upper end of the glass transition) is dependent on the degree of phase separation between the hard and soft segments. In products with large amounts of hard segment (>50%), the mobility of the soft segment is considerably reduced. As a result, cold flexibility properties are impaired. Tensile strength, 300% modulus and tear strength are substantially affected by the melting point (TM) of the soft segment. Increasing chain length of the soft segment and decreasing amounts of hard segments, as well as high linearity of the PU favor crystallization.
The viscous-elastic behavior of segmented linear PU elastomers was investigated through modulus/temperature experiments. Similar properties to those observed for block elastomers (e.g., a butadiene/styrene block copolymer) such as an extensive plateau in the high modulus area were also found for segmented linear PU elastomers. The absence of hydrogen bonds in hydrocarbon elastomers leads to the conclusion that hydrogen bridges are not the sole responsible for the observed properties in PU elastomers. In the two systems, physical interactions reinforce the structure until the melting temperature of high modulus blocks is reached. Through selective solvation of aromatic PUs and segmented polyester with different solvents, it was possible to demonstrate that an association of hard segments in the solid state (hard segment domains) is a prerequisite for the existence of a high transition temperature.
As previously mentioned, in segmented PU elastomers hydrogen bridges are formed between active hydrogen atoms of -NH- urethane groups and -CO- urethane groups, with polyols polyester -CO- groups, and more difficultly with the oxygen atom of polyol polyethers. Infrared investigations of N-H stretching-related adsorptions indicate that more than 90% of the N-H urethane group hydrogen forms hydrogen bonds. On the other hand, a study related to urethane -CO- groups shows that only about 60% of these groups are linked or associated. This indicates that a substantial portion of hydrogen bonds occurs between -NH- urethane groups and -CO- groups of the polyester soft blocks.
Other studies show that, for polyester-based PU elastomers, the formation of hydrogen bonds depends on the size of the polyester soft segment. In polymers with high urethane concentration, hydrogen bridges between N-H groups and urethane -CO- groups are more frequent. Though, when urethane concentration is reduced, connections between N-H urethane groups and polyester -CO- groups become more important. Spectroscopic infrared analyses of polyester and polyether systems indicate that in polyester systems hydrogen bonds are formed mainly with polyester -CO- groups, while in polyether systems, such bonds are formed with urethane -CO- groups.
Drastic modifications in PUs properties can be introduced by varying the crosslinking degree. Reticulations may be formed by reaction of the isocyanate excess with urea or urethane groups yielding biuret or allophanate cross-linking, or by using tri or poly-functional alcohols or amines as chain extenders. Whereas elongation and permanent set decrease with increasing crosslinking density, tension strength initially increases, but later on decreases. When a predominantly linear segmented PU is reticulated, physical and chemical crosslinking effects overlap. However, if the polyaddition reaction is carried out directly in presence of tri- or higher functional polyisocyanate or polyols, which lead to an early network of primary valences, the formation of physically crosslinked areas (domains) can be prevented. Hence at low chemical crosslinking, modulus of elasticity decreases. Under extreme conditions, due to the fact that chains are already fixed, segregation will not occur, and even temperature treatment (annealing) will not result in an improved property level.
The effect of crosslinking density on physical properties of PUs elastomers is shown by data (Table 1.13) obtained by the substitution of 1,4-butane-diol for trimethylol propane for, as chain extender of PU based-polyester produced with MDI and ethylene glycol polyadipate. These data show the molecular weight increase of crosslinking units (Mc) (or decrease in crosslinking density) with the triol amount in the reagents. The initial decrease in modulus with the increase in crosslinking degree is opposite to the results observed in conventional polyhydrocarbon elastomers, where an increase in the reticulation corresponds to a modulus increase. With PUs, it happens that a larger crosslinking number reduces the chain orientation and formation of hydrogen bonds or other intermolecular interactions. This phenomenon prevails until the crosslinking density is sufficiently strong to exert its own effect towards increasing PU modulus.
Table 1.13 - Effect of crosslinking density (Mc) on PU physical properties|
Mc
|
Tensile strenght
(MPa)
|
Elongation at break
(%)
|
Stress at 100% strain
(MPa)
|
Tear resistence (kN/m)
|
Hardness (Shore B)
|
Tensile set
(%) |
Compression set
(%) |
|
2100 |
12,4 |
170 |
3,9 |
5,4 |
57 |
0 |
1,5 |
|
3100 |
12,0 |
200 |
3,0 |
4,5 |
53 |
0 |
16 |
|
4300 |
10,0 |
280 |
2,1 |
5,4 |
49 |
0 |
10 |
|
5300 |
19,3 |
350 |
1,9 |
5,4 |
46 |
0 |
0 |
|
7100 |
31,0 |
410 |
2,3 |
7,1 |
51 |
0 |
25 |
|
10900 |
38,6 |
490 |
3,2 |
10,8 |
55 |
5 |
40 |
|
21000 |
38,0 |
510 |
3,5 |
15,0 |
56 |
10 |
45 |
|
infinite |
46,5 |
640 |
4,3 |
54,0 |
61 |
15 |
55 |