Organometallic catalysts (Table 2.5) promote the polymer forming or gellation reaction between the isocyanate and a polyol. Of the many metals available, tin compounds like stannous octoate (SnOct) and dibutyltin dilaurate (DBTL) are the most popular. Stannous octoate is used in most flexible foam systems, except pre-blended two component ones where its low hydrolytic stability is unacceptable. Low levels of DBTL often catalyze microcellular elastomers, RIM systems and cast elastomers.
Table 2.5 - Organometallic catalysts|
CATALYST |
MAIN APPLICATION |
|
Stannous octoate |
Polyether based slabstock and moulded flexible foams catalysis. |
|
Dibutyltin dilaurate |
Microcellular, RIM and cast elastomers catalysis. |
|
Potassium acetate |
General purpose catalyst. |
|
Potassium octoate |
Isocyanate trimerization catalyst. |
|
Dibutyltin mercaptide |
Hydrolysis resistant catalyst. |
| Dibutyltin thiocarboxylates | Delayed action (hindered) catalysts for RIM and HR foams. |
|
Phenylmercuric propionate |
In glycol solution for potting compounds, as a powder for delayed action catalysis. |
|
Lead octoate |
Chain extension catalysis. |
|
Alkaline metal salts, (K2CO3, NaHCO3 and Na2CO3) |
General catalysts for urethane reaction and for isocyanate polymerization. |
|
Calcium carbonate |
Filler with catalytic effect. |
|
Ferric acetylacetonate |
Catalyst for cast elastomer systems. |
Numerous studies have shown that organo tin catalyzed urethane reactions do not follow first order kinetics, and that the organo tin catalysts promote a number of side reactions, acting synergistically with at least one tertiary amine (TEDA). The catalytical activity of tin compounds can be significantly increased by addition of amines (Table 2.6 and Figure 2.7).
Table 2.6 - DBTL / TEDA Synergy|
DBTL |
TEDA |
Activity order |
|
0,0 |
0,0 |
1 |
|
0,0 |
0,3 |
330 |
|
0,3 |
0,0 |
340 |
|
0,3 |
0,3 |
1780 |
DBTL and TEDA, when used as sole catalysts, exhibit lower catalytical activity than if used together. Compared to the activity of the sole catalysts, and when both catalysts are combined, the activity increases by a factor 5. Similar activity increases are observed with SnOct and TEDA.

Figure 2.7 - DBTL and TEDA synergism
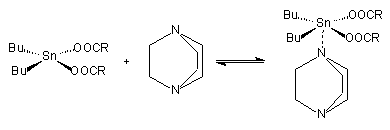
Organo metallic catalysts act as Lewis acids, and are generally thought to function by interacting with basic sites in the isocyanate and polyol compounds. They form an intermediate complex with an isocyanate group and a hydroxyl group of the polyol. This complex formation is inhibited by steric hindrance of the metallic atom. This steric effect is used in one type of delayed action catalyst; i.e. one that is not very active at room temperature but becomes so when the reaction temperature rises. There are three complementary mechanisms for activated complex formations. One conceptual mechanism involves activation of isocyanate molecules. Polyol attacks this complex at the isocyanate carbon atom to again propagate the polymer and regenerate de catalyst (Figure 2.8). In other mechanisms, the polyol is activated by the formation of a complex with the organometallic catalyst. This adduct can react with isocyanate to give a carbamate, which further reacts to additional polyol propagating the polymer and regenerating the catalytic species. The final mechanism attempts the synergism (Figure 2.7) between organometallic compounds and amine catalysts.
Figure 2.8 - Activation of isocyanate molecules by organo-metal.
The principal tin catalyst used in the manufacture of flexible PU foam is stannous octoate [Sn(C8H15O2)2]. This is an inorganic tin catalyst produced from tin metal and 2-ethylhexanoic acid. When used in combination with tertiary amines, this material affords an excellent balance of cost performance to provide the reaction profiles required by today's high volume flexible slabstock production lines. Tin can take on two oxidation states Sn2+ (stannous), which are found in SnOct and Sn4+ (stannic) found in organo tin compounds. Catalysts, like DBTL, have more a pronounced catalytic effect in the polymerization reaction between the isocianato and the alcohol than in the expansion reaction between the isocianato and the water. On the other hand, catalysts, such as the stannous octoate, the base of which is Sn2+, also present catalytic effects in the reaction of expansion of the isocianato with the water. Grignard reactions are used to produce the organo tin intermediates di-n-butyl tin oxide and di-n-butyl tin chloride. A wide range of organic acids reacted with this basic structure to produce compounds like DBTL (Figure 2.9). In addition, di-butyl tin can be reacted with dibasic acids, as maleic acid, and with mixtures of monobasic acids to provide numerous homologues.
Catalysts, like DBTL, have more a pronounced catalytic effect in the polymerization reaction between the isocianato and the alcohol than in the expansion reaction between the isocianato and the water. On the other hand, catalysts, such as the stannous octoate, the base of which is Sn2+, also present catalytic effects in the reaction of expansion of the isocianato with the water. Grignard reactions are used to produce the organo tin intermediates di-n-butyl tin oxide and di-n-butyl tin chloride. A wide range of organic acids reacted with this basic structure to produce compounds like DBTL (Figure 2.9). In addition, di-butyl tin can be reacted with dibasic acids, as maleic acid, and with mixtures of monobasic acids to provide numerous homologues.
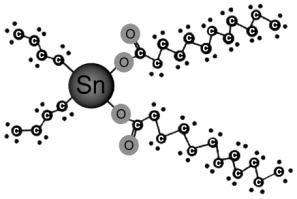
Figure 2.9 - Basic structure of di-n-butyl tin dilaurate (DBTL)
For CASE applications, DBTL can be considered the workhouse catalyst. It is efficient; i.e., a very low level of catalyst will greatly increase the NCO/OH reaction rate. However, as with any catalyst, certain problems may be encountered, which may include issues of stability-reactivity, hydrolysis of ester groups, catalysis of the water/isocyanate reaction and environmental concerns. The process of selecting a catalyst depends on several factors. Addition of metal catalysts in concentrations that can be measured in ppm have a profound effect on the reaction rate; yet, very low concentrations of impurities can also effect the reaction rate. Often resins, additives and pigments contain impurities, which can interact with catalysts and deactivate them, or the impurity itself can act as a catalyst. Catalyst deactivation can also be a function of water content or acid number of the resins. In addition, impurities have different effects on different metal catalysts. Furthermore, catalyst selection will also depend on the desired properties, and pot life requirements. DBTL and dibutyltin diacetate (DBTA) are very versatile catalysts for the NCO/OH reactions. Moreover, a range of organotin free catalysts includes bismuth, aluminum and zirconium, which are environmentally more acceptable and offer performance advantages.
Bronsted or Lewis acids retard the proton transfer to the isocyanate groups. Common inhibitors are HCl, benzoylchloride and p-toluene sulfonic acid and others added in the ppm region to the isocyanate. These materials play an important role in the preparation of prepolymers based on highly reactive polyols or amines and isocyanates.